Background
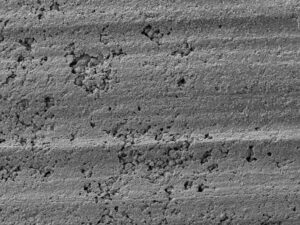
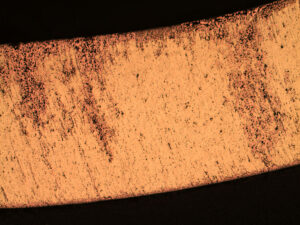
Two 90-degree brass plumbing fittings were received by the Rimkus Materials Testing and Investigation team for a corrosion investigation to determine the extent of deterioration and leakage. The fittings were reported to have been installed in an occupied residential home during a two-year restoration project. Excessive corrosion deposits, stains, and discoloration were visually observed on the outer surfaces of the fittings.
Services Provided
To evaluate the cause of corrosion, the brass plumbing fittings were subjected to a series of testing and analytical techniques including visual and stereoscopic examinations, scanning electron microscope (SEM) examinations, elemental analysis, metallography/microstructure evaluation, chemical composition analysis, and hardness testing.
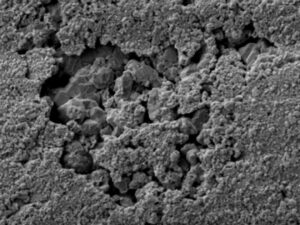
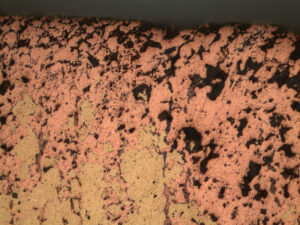
Results indicated the fittings were severely degraded due to “plug-type” dezincification corrosion. The dezincification was due to the high concentration of internal deposits, hot water temperature, water pH, water chloride content, stagnant or low flow conditions, and the use of a brass material susceptible to this form of corrosion (copper alloy C36000, leaded brass).
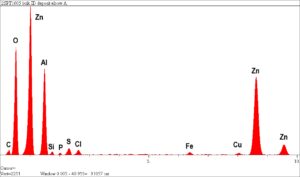
Zinc was found to be completely leached from the brass alloy (normally +/-34% zinc), leaving a porous matrix through the entire wall thickness of the fitting. Leakage was determined to be imminent at all locations with comparable fittings in the hot water line where similar flow velocities were found.
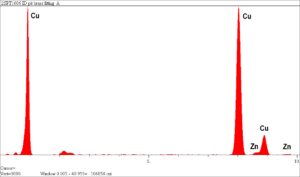
Rimkus experts performed energy dispersive x-ray spectrographic (EDS) microanalysis to identify the elemental composition of localized corrosion cells that occurred as a result of heavy internal deposits, which was determined to be zinc oxide. The corrosion-attacked regions were essentially made up of pure metallic copper, with only traces of zinc remaining. The heavy internal deposits were also mainly zinc oxide, due to the corrosion, as well as aluminum oxide. The source of aluminum oxide, without significant levels of calcium carbonate or silicon oxide, was unusual and may indicate corrosion of an aluminum component in the hot water system.
Conclusion
The Rimkus team recommended that all similar fittings should be replaced with dezincification-resistant brass alloy fittings. In addition, it was recommended that the home’s water be tested and treated for pH and chlorine content, and that the maximum hot water temperature be decreased to help reduce corrosion. Additionally, any aluminum components in the hot water system should also be inspected for corrosion damage.
Photos